Introduction
We have created a system to produce NMN using something called cell-free metabolic engineering, or CFME. CFME has existed for several decades, but until now it has only been used as a tool in research to understand metabolic pathways.
What is CFME?
Nearly all reactions that happen inside all living things are facilitated by enzymes. Enzymes are proteins made by the cells of an organism to change molecules from one form to another.
Even though most enzymes only make one specific change to very select starting molecules, living things are able to string several enzymes together so one produces the starting compound of the next.
These chains of enzymes are called metabolic pathways and they can change even the smallest organic molecules into the largest and most complex molecules in nature by making one minor change at a time, one enzyme at a time.
Cells use hundreds of metabolic pathways composed of thousands of enzymes to produce nearly everything a living creature is made of.
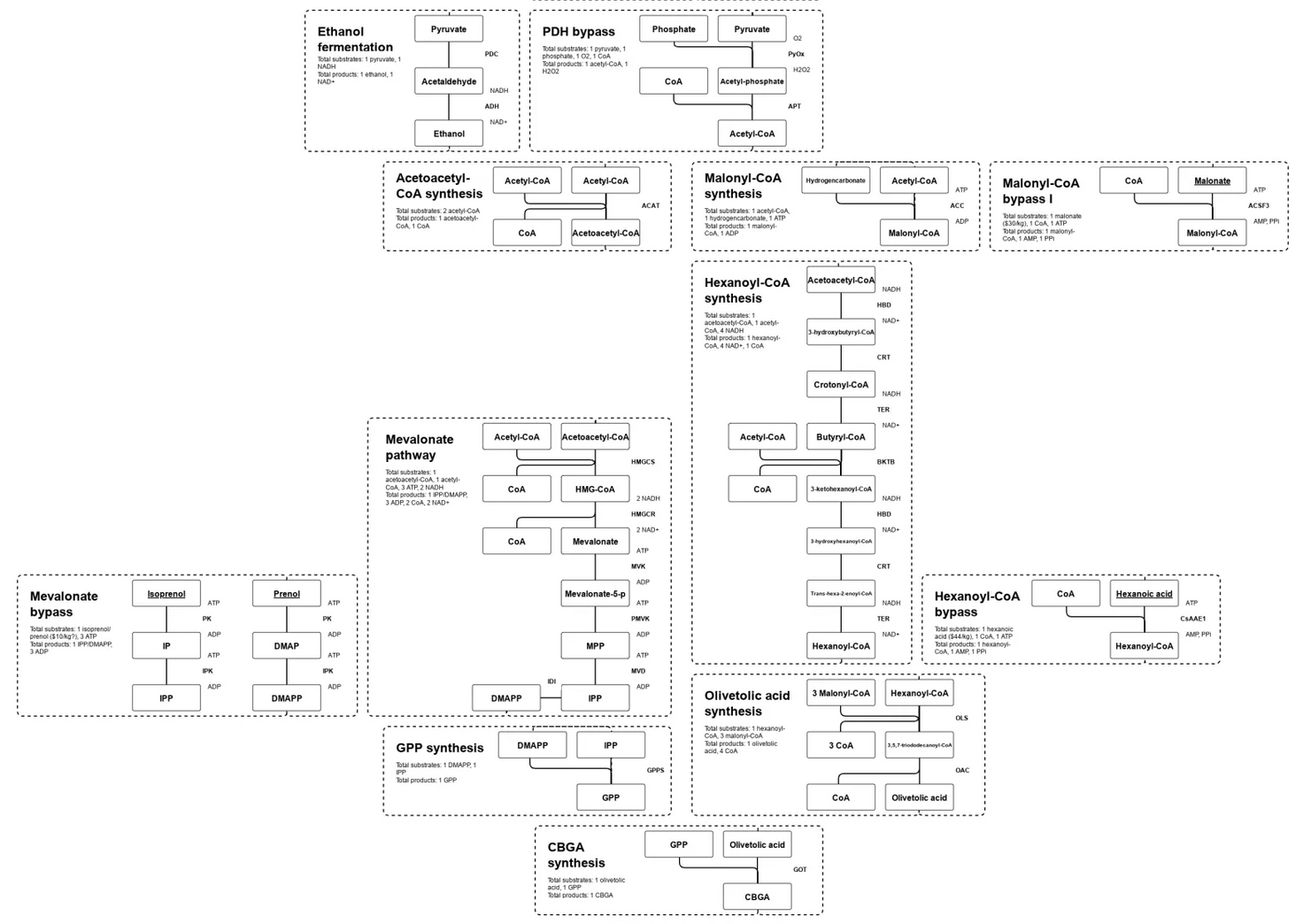
The idea of CFME is that if you take an enzyme out of a cell and put it in a tube with the right ingredients, it will do its job the same or even better than it did inside the cell.
Historically, this concept has been used as a powerful research tool to study the properties of each newly discovered enzyme and understand how metabolic pathways work.
However, it can also be used as a sort of natural molecular manufacturing process, just as cells use enzymes to manufacture specific molecules.
By isolating enzymes and assembling them into specific pathways, you can “distill” that natural molecular manufacturing power to produce huge amounts of one end product with amazing efficiency.
By assembling different pathways from the huge variety of organisms on the planet, you could produce literally any compound made in nature.
Why hasn’t CFME been used for production before?
The potential of CFME has been recognized almost since its conception. It has been referred to as the holy grail of biomanufacturing, with many groups across the world contributing unique approaches to a successful production-scale system.
However, such a system has remained elusive for over two decades because of two critical problems with the technology: The enzyme problem and the cofactor problem.
The Enzyme Problem
As mentioned earlier, all organisms must produce enzymes in order to live. Enzymes are one of only a few fundamental pillars of all life on earth. But different enzymes are produced at very different rates and their locations within cells are tightly regulated.
Extracting and isolating the necessary amount of each enzyme from its native organism would be nearly impossible, let alone even close to efficient. Luckily, a technology which allows you to produce proteins (including enzymes) from one organism in the cells of another has existed almost since the birth of molecular biology as a field.
It’s called recombinant protein expression, and today it’s used almost universally in molecular biology labs throughout the world. Unfortunately, even now, decades after its first use, this technology is still being refined; every individual protein and every expression system present their own unique, often unknown challenges.
Achieving any production of a target protein in most organisms is a challenge in itself, but vastly more difficult is achieving the very high efficiency production required to supply a production-scale CFME system with enzymes. Herein lies the enzyme problem: It is usually very expensive to supply a production-scale CFME system with enzymes.
Our solution to the enzyme problem
While other groups have accepted the increased production costs contributed by inefficient enzyme supply, we have developed a new protein production technology more efficient than any others in existence today.
We use a slime mold called Physarum polycephalum that is capable of growing to enormous density in a short time. We developed nearly half of the molecular biology toolbox available for Physarum polycephalum.
We also created the current best solid state bioreactor design in which to grow it. After over half a decade of development, the Physarum polycephalum expression system now blows traditional systems out of the water, allowing us to produce enzymes with little upfront individual development cost and very minimal continuous operating cost.
Why NMN?
We chose NMN as our first product to bring to customers because it is already widely used at relatively low doses and, in most cases, increasing intake would directly increase benefits, but most people don’t because it would be prohibitively expensive. One of our primary missions is to make the most impactful natural health products available on a budget.
Enzymatic synthesis of NMN is currently the best production method, but it’s also the most expensive because it’s only a single enzyme conversion and the inputs are expensive. Our system represents the first advancement that allows many enzymes to be strung together, which enables the use of much lower price inputs because we can build the molecules from the ground up like living cells do.
Even though our production scale is comparatively very low at the moment as we ramp up, we are still able to offer fully purified, encapsulated, and bottled 1-2 month supplies of high dose NMN for half to a third the price of most other NMN products available. And we expect to increase that difference as we scale up our system.